Identity.
Einsteinium, element 99, is a man-made marvel born from a blast. Named
after the genius physicist, it shines with radioactivity, a silvery
ghost residing in the actinide family. Forget finding it in nature;
this fleeting element exists in mere milligrams, produced in nuclear
reactors or the debris of hydrogen bombs. While short-lived, its
significance is far from small. Einsteinium serves as a key
researcher's tool, helping scientists unlock the secrets of the atomic
nucleus and explore the uncharted territory of superheavy elements. In
essence, it's a rare yet vital puzzle piece in our quest to understand
the universe's building blocks.
Atomic Structure:
Einsteinium is a Block F, Group 3, Period 7 element. The number of
electrons in each of einsteinium's shells is 2, 8, 18, 32, 29, 8, 2
and its electronic configuration is [Rn] 5f11 7s2. The einsteinium
atom has a radius of 186.pm. In its elemental form, einsteinium's CAS
number is 7429-92-7.
History.
The story of einsteinium begins not in a lab, but with a bang. In
1952, the first hydrogen bomb was detonated on Enewetak Atoll,
unleashing unimaginable power. When the dust settled, scientists
ventured into the radioactive aftermath, collecting debris for
analysis. In a lab at Berkeley, California, a team led by Albert
Ghiorso made a surprising discovery: amidst the irradiated particles,
nestled a new element, number 99.
Born from the intense neutron bombardment within the bomb, this
element was christened "einsteinium" in honor of the renowned
physicist's groundbreaking work on relativity and nuclear energy.
However, identifying einsteinium was no easy feat. Only 200 atoms
were initially found, requiring meticulous chemical separation and
radioactive detection techniques. The discovery remained
classified until 1955 due to the sensitive nature of nuclear
research during the Cold War.
While initial einsteinium samples were microscopic, scientists
gradually developed methods to produce larger quantities through
targeted neutron irradiation in dedicated reactors. This allowed
for further study of its properties, revealing a soft, silvery
metal with fascinating properties. Notably, einsteinium's
radioactivity makes it invaluable in scientific research,
particularly in investigations of superheavy elements and the
understanding of atomic nuclei. Though rare and challenging to
work with, einsteinium remains a testament to human ingenuity and
serves as a key player in unraveling the universe's secrets.
Usage.
Einsteinium, though rare and radioactive, serves as a scientific
powerhouse. It helps unlock the secrets of the nucleus by creating
superheavy elements, fuels curiosity in studies of spontaneous
fission, calibrates high-precision instruments, and even indirectly
powers deep-space exploration through its role in the production of
long-lasting power sources. Though not something you'll find in
everyday life, its contributions in research are far from small.
-
Unravelling the Atomic Nucleus: Due to its intense
radioactivity, einsteinium serves as a powerful tool for probing
the structure and behavior of atomic nuclei. Scientists bombard
other elements with einsteinium nuclei to create even heavier,
more exotic elements, pushing the boundaries of the Periodic Table
and our understanding of nuclear stability.
-
Fueling Scientific Curiosity: Although not abundant enough
for practical applications, einsteinium plays a crucial role in
various scientific studies. Its unique radioactive properties
allow researchers to investigate phenomena like spontaneous
fission, where an atomic nucleus splits apart on its own. This
data helps refine our understanding of nuclear processes and their
potential risks or applications.
-
Cailbratiing Instruments: The consistent and well-defined
emission of alpha particles from certain einsteinium isotopes
makes it valuable for calibrating high-precision radiation
detectors. These detectors are essential in a variety of fields,
from nuclear medicine to environmental monitoring, ensuring their
accuracy in measuring different types of radiation.
-
Space Exploration (Indirectly): While einsteinium itself
isn't used in spacecraft, it plays a role in the development of
certain radioisotope thermoelectric generators (RTGs). These
long-lasting power sources rely on the heat generated by the
radioactive decay of elements like plutonium, some of which are
produced by bombarding lighter elements with neutrons, a process
that can sometimes yield tiny amounts of einsteinium as a
byproduct. These RTGs provide reliable power for deep-space
missions, powering probes exploring distant corners of our solar
system.
Some of the benefits of using Einsteinium are:
-
By bombarding other elements with einsteinium nuclei, scientists
can create even heavier, more exotic elements, expanding our
understanding of atomic structure and nuclear stability. This
knowledge has implications for fields like nuclear physics and
astrophysics.
-
Einsteinium's unique radioactive properties allow researchers to
study phenomena like spontaneous fission, where an atomic nucleus
splits apart on its own. This data helps inform our knowledge of
nuclear decay and fission processes, which have applications in
areas like nuclear power generation and waste management.
-
Certain einsteinium isotopes emit alpha particles with consistent
and well-defined energies. This makes them valuable for
calibrating high-precision radiation detectors used in various
fields, such as nuclear medicine and environmental monitoring,
ensuring accurate measurements of different types of radiation.
-
While not directly used in spacecraft, einsteinium can be a
byproduct in the production of certain radioisotope thermoelectric
generators (RTGs). These long-lasting power sources rely on the
heat generated by the radioactive decay of elements like
plutonium, and some production methods involve neutron bombardment
that can yield tiny amounts of einsteinium. These RTGs provide
reliable power for deep-space missions, allowing probes to explore
distant regions of our solar system.
Sources.
Forget mining for einsteinium! This elusive element isn't found in
nature's treasure chest. Instead, it's born in the heart of
high-powered nuclear reactors, where targeted neutron bombardment
transforms other elements like plutonium into this rare gem. Traces
were even detected in the fallout of early hydrogen bomb tests,
highlighting its unique birth process. While challenging to produce,
these minuscule quantities fuel scientific discoveries, pushing the
boundaries of our understanding of the atomic world.
Properties.
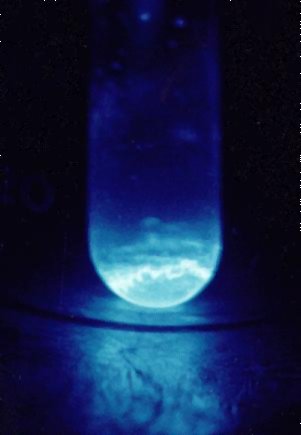
Glowing Rockstar: Due to its intense radioactivity, einsteinium
exhibits a visible glow, making it the only element on the periodic
table readily observable with the naked eye (in specialized
facilities, of course!). This glow results from the energy released
during its radioactive decay.
Slivery Shape-Shifter: This soft, silvery metal exists in
different crystalline forms depending on temperature and pressure. It
readily changes its internal structure, showcasing its adaptability.
Reactive Renegade: Like other actinides, einsteinium is highly
reactive, readily forming compounds with oxygen, water, and acids.
This reactivity necessitates its careful handling and study in
specialized environments.