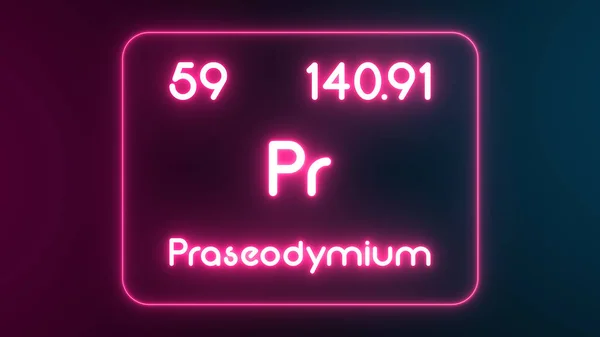Praseodymium
Praseodymium (Pr), a silvery-white member of the lanthanide family, isn't your average metal. While soft and malleable, it oxidizes readily in air, turning a vibrant green. Despite its reactivity, praseodymium's unique properties shine in many applications.
Praseodymium's magic lies in its ability to imbue materials with vibrant hues. Its salts paint glasses, enamels, and glazes a stunning yellow, while didymium glass, containing praseodymium, filters infrared radiation, protecting welders and glassmakers.
But praseodymium is more than just a pretty face. It strengthens magnets, improves certain alloys, and even finds uses in ceramics and lasers. Though not essential for life, praseodymium adds a touch of brilliance to our world, proving that sometimes, the rarest elements hold the most potential.